Light as energy
Light and colour, light and energy:
Light is a form of energy that is transported as electromagnetic radiation but interacts with material particles as discrete packets of energy that are called photons. How do we know?
Sun provides us with heat and light. This travels through the near vacuum of space. So there is no material to transport energy. When we block light we also block the heat radiation. Any source of heat also radiates some visible light. Heat a piece of iron and the iron changes colour from a dull grey to red to yellow to white when light of all wavelengths are radiated in addition to the heat. On a cold day the temperature inside a glass house is much higher – why? Because light of a shorter wavelength can travel through glass and is absorbed by the ground and plants. The ground reflects back radiation of a higher wavelength – infra-red and heat radiation. This cannot pass through glass and so the glass house is much warmer. Keep a black cloth under light and it will be distinctly warmer after sometime.
What can we infer
- Energy is transported from a source to a destination without a medium
- The energy is of different kinds – heat, light of different colours, X rays etc
- One form of the energy can get converted to another. They must therefore have things in common - radiant heat and light are similar in many ways and different in some ways.
- Studies done on how light travels has helped us visualise it now as changing electrical magnetic fields that interact with other material objects as packets of energy.
Theory of Light
- This radiation can be thought of as waves of different wavelengths. The waves are not of material objects but travelling waves changing electric and magnetic fields. Different wavelengths are associated with different energy levels and different kind of radiation. (we will see more about this when we look at light as a particle) The electromagnetic spectrum consists of wavelengths varying from hundredths of a nanometre to hundreds of metres. Visible light falls within the wavelength range of 400 to 800 nm (1 nanometre = 10-9m) and is a small part of the electromagnetic spectrum. Blue light has the smaller wavelength – around 400 nm and red light is around 800 nm. Ultra-violet rays, X rays and gamma rays are more energetic and shorter wavelengths (more later!) and infra red, heat radiation and radio waves are longer wavelengths and lower energy levels.
- It is the peculiar nature of sub-atomic particles that they can be treated as particles – in interactions - or as probability waves when we are considering location in space. In the case of light we find that we can associate it with particles – called photons. These are often also known as messenger particles and current theory has it that the movement of energy occurs by the creation and destruction of these virtual particles starting from the source of the energy till the point when it reaches the user of the energy. Different photons have different wavelengths associated with them. Each photon can be treated as a packet of energy and the amount of energy associated with the particle is given by the equation Energy E = hυ where υ is the frequency associated with the wavelength and h is the Planck's Constant. (Remember that the velocity of a wave is given by c = υλ where λ is the wavelength of the associated wave). For more discussion on the speed of light, please click here. This means that there is a wavelength associated with the photon particle and this is the wavelength we refer to when talking about the wavelength of light.
Since wavelength is inversely proportional to the frequency we can see that the shorter wavelength photon has the greater energy. What this means is that the smallest unit of energy associated with each wavelength is fixed this is the energy of 1 photon of that wavelength. Of course, if there are more photons then there is more energy. But more energetic photons can penetrate matter more than less energetic photons – that is why X rays are not stopped by flesh and muscles but only by bones but light gets bounced off the human body.
Light and Colour
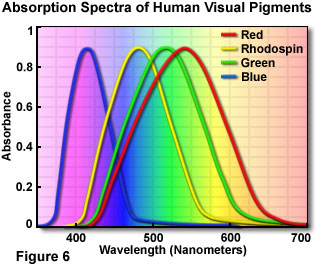
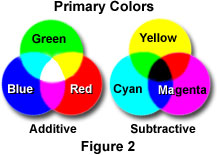
- If light is made up of particles called photons and different wavelengths are associated with different photons of different energies then how do we make out the difference? With light, the way we distinguish them is by the sense of colour. We associate different colours with different wavelengths. The nature of perception will be dealt with later but it would be good to look at some aspects of it now. Perception is dependent on the physiology of the eye and our eye is sensitive to light, its absence and the various shades in between. It is also sensitive to three colours. That is to say we have nerve endings in the eye that can measure the intensity of the light - these are known as rods. We also have three other types of nerves- all called cones - that respond maximally to three different wavelengths. The rods respond best to wavelengths in the 500 nm range. Rods have a wider range of response than the cones. The extent of response of the cones will depend on the wavelength. Light which consists mostly of wavelengths around 430 nm it will be seen as blue. If it is around the 540 nm range it would be seen as green and if it is around 600 nm it would be seen as red. If the wavelength is somewhere between 540 and 600 nm then both the specialised cones will be excited to a limited extent and this will produce a sense of yellow. Interestingly, if we were to shine light of wave lengths 540 & 600 nm we would still produce a sense of yellow as the two cones which are sensitive to these wavelengths are excited. The fact that more than one type of cone is excited implies that colour vision is additive and red, green and blue are called the Additive Primary Colours.
As you can see in the adjacent figure when all three additive colours appear together we get white. Blue and Green together give the sense of Cyan, Blue and Red a sense of Magenta and Green and Red gives Yellow. What happens if we remove Red from white light? We get Cyan – the combination of Blue and Green. Similarly, Yellow and Magenta are produced by removing Blue and Green respectively. That is why these three colours are called Subtractive Primary colours – they are produced by removing one colour from white light. In the figure above you can see how the additive primary colour is produced by mixing different
subtractive primary colours – only the common colour is visible when two subtractive colours are mixed – green is produced when cyan and yellow are mixed etc. When you mix all three the result is Black.
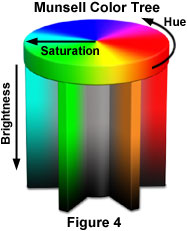
This by itself does not explain all the colours that we see. For example colours like brown do not exist in the combination of the primary colours. For this we need to add additional dimensions. Every colour that we see is actually a combination of a number of wavelengths. The rim of the disk in the adjoining figure shows the range for the primary colour combination. This is what we know as ‘Hue’. The ratio of the dominant or primary wavelength to the other wavelengths
defines the parameter ‘saturation’ which moves you radially along the disk from the centre for maximum saturation at the rim for each of the colours in the combination. The last parameter is the ‘brightness’ – the intensity or energy associated with different wavelengths - and is shown as the trunk of the tree in the accompanying diagram. You can see that by varying the three parameters we can get the colour that we want – including colours like brown and grey.
All this discussion is in relation to mixing of light. When we mix pigments the process is very different. The primary pigment colours are red, yellow and blue. In the case of pigments only specific wave lengths of the white light that is incident on the pigment are reflected. So red pigment will predominantly reflect red light, yellow light will predominantly reflect yellow and so on. When we mix blue and yellow pigment we get green, red and yellow gives us orange, red and blue gives us purple and so on.
Key vocabulary
- Light – the kind of energy that allows us to see
- Electro magnetic radiation - The way light energy travels
- Photon – The packets of energy that light consists of
- Wavelength – a property associated with different colours. We can try and explain this in greater depth when we work on the sound module.
- Additive Primary Colours – the colours of light to which our eyes are sensitive and which together combine to form white light.
- Subtractive Primary Colours – the colours produced when one of the additive primary colours is removed from white light.
- Hue, Saturation and Intensity – The three parameters that allows us to see all the different colours including colours that are not part of the VIBGYOR spectrum.
Additional web resources
For this section also see the following : Olympus Microscopy Resource Center Physics of Light and Color
- www.olympusmicro.com/primer/lightandcolor/primarycolorsintro.html
- www.olympusmicro.com/primer/java/primarycolors/additiveprimaries/index.html
- www.olympusmicro.com/primer/java/primarycolors/subtractiveprimaries/index.html
These web sites will also allow use of some interactive programs on-line and that will clarify things further.