Salts and solubility
Revision as of 16:35, 11 September 2015 by ashok (talk | contribs) (→Activity No # 1 - salts and solubility)
Activity No # 1 - salts and solubility
salts and solubility
Shared by:
Triveni shetty
Estimated Time
5mint
Materials/ Resources needed
- computer,
- screen,
- electricity,
- projector.
Prerequisites/Instructions, if any
observe the solubility of salts carefully.
Multimedia resources
PhET
Website interactives/ links/ simulations
file:///opt/PhET/en/simulations/category/new.html
Process (How to do the activity)
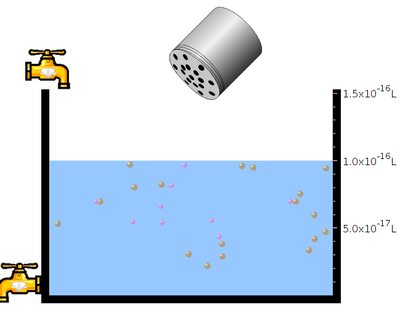
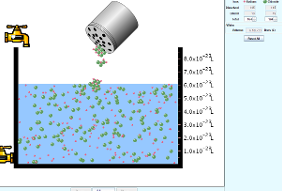
- Click the “tap” to pour the water and take required amount of water.
- Click “table salt” button.
- Click and Shake the “bottle” to add little amount of sodium chloride salt.
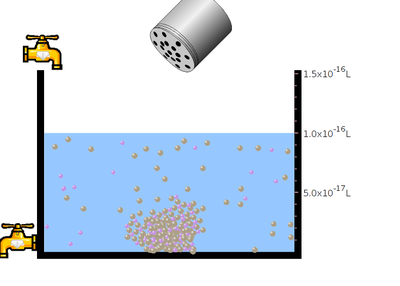
- Observe the solubility of salt in water. In the next step click the “reset all”botton. Then click the bottle to add mercuric bromide salt. Again observe the solubility.
Developmental Questions (What discussion questions)
- what are the basic needs of living being?
- Name the sources of water?
- which source of water is suitable for domestic purpose?
- Ocean or sea water is not suitable for this purpose. why?
- Sea water is salty. Why?
- What happened when sodium chloride is added to water?
- What happened when Mercuric chloride is added to water?
- Between two salts which dissolves faster?
Evaluation (Questions for assessment of the child)
- What is the chemical name and molecular formula of common salt?
- Name the elements present in common salt ?
Question Corner
- Name the universal solvent? Why?
To link back to the topic page Give the link of the page name from where activity was given Back
Activity No # 2 - salts and solubility
Shared By:
Mamtha Poojari
Estimated Time
8 min
Materials/ Resources needed
- Computer,
- monitor,
- mouse,
- Projector,
- PhET simulations.
Prerequisites/Instructions, if any
Observe the changes care fully and note down those changes.
Multimedia resources
Website interactives/ links/ simulations
file:///opt/PhET/en/simulation/soluble-salts.html
Process (How to do the activity)
- Open PhET simulation called salt and solubility.
- Click on the tap to fill the water up to 5 liters.
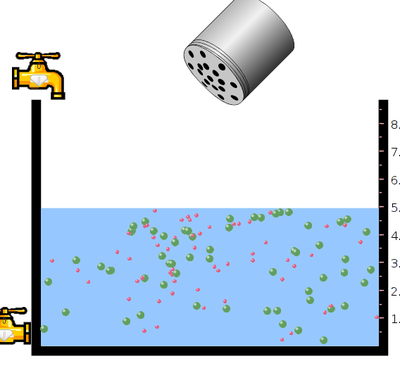
- Select the salt NaCl from the menu and click on the salt bottle to add the salt into the vessel containing water by shaking the bottle.
- Now click on the tap present at the bottom of the vessel to remove water . click and shake the salt bottle to add little more salt.
- Again click and shake the salt bottle to add more and more salt, now observe the solubility.
- Click on the tap to add 5l of water in to the same vessel.
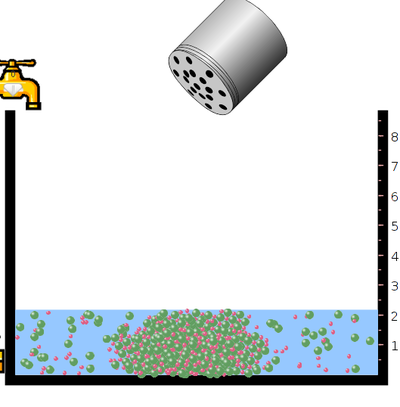
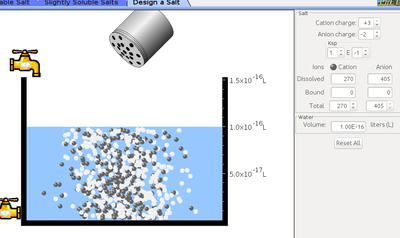
- Click on the slightly soluble salt and select mercury bromide.
- Click on salt bottle to increase or decrease the concentration of mercury bromide into the vessel by shaking the bottle.
- Click on design a salt menu and adjust cation and anion charge.
- Click on salt bottle and shake to add designed salt to the vessel.
- Click on the tap to remove or to fill the water into the vessel.
Developmental Questions (What discussion questions)
- What changes you observe when salt is added to the vessel?
- what you observe when remove water and more salt into the vessel?
- when you add water to the same vessel what happens to the solubility the table salt?
- what happens to the solubility when mercury bromide is added to the vessel containing water?
- What do you observe when you increase or decrease the concentration of mercury bromide and water?
- What are the changes you observed when you added your own designed different types of salts?
Evaluation (Questions for assessment of the child)
- What is solvent?
- What is solute?
- What is solution?
- When does a solution is said to be saturated?
Question Corner
- What is universal solvent?
- What is the importance of the solubility of ions in our body?
To link back to the topic page Give the link of the page name from where activity was given Back